Neutrophils are crucial innate immune cells which protect the host by killing infectious pathogens. Single cell RNA-sequencing analyses have previously described transcriptional heterogeneity of neutrophils and precursors, however the genome regulatory events underlying the transcriptional changes are less well characterised. Understanding this is important as neutropenia can be caused by dysregulation of the genome regulatory events that orchestrate neutrophil maturation. A good example is the use of immunomodulatory drugs (IMiDs, lenalidomide [LEN] & pomalidomide [POM]) for the treatment of multiple myeloma (MM) which are now standard-of-care, but their use is frequently complicated by neutropenia because of neutrophil maturation impairment. This is due to Cereblon (CRBN)-driven substrate ubiquitination and Ikaros degradation, causing PU.1 downregulation. However, restoration of Ikaros levels cannot fully alleviate the IMiD-induced myeloid differentiation block suggesting other mechanisms might also contribute.
Our aim was to build a multiomic atlas of human myelopoiesis and use this to interrogate the molecular drivers of IMiD-induced neutropenia.
We developed an ex vivo myeloid cell differentiation assay using human mobilized peripheral blood CD34 + cells from healthy donors and optimised methods for single cell multiomic (combined RNA-seq & ATAC-seq) analysis. This platform was validated orthogonally (immunophenotype, morphology & functional) and employed to construct a multi-omic single-cell landscape of normal human neutrophil differentiation and it's perturbation by IMiDs.
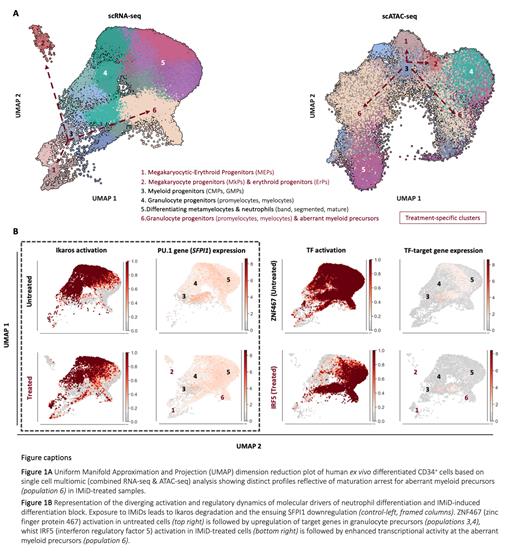
Single cell transcriptomes from >110,000 cells from untreated and IMiD-treated samples were analyzed, capturing the full spectrum of human myelopoiesis spanning from early myeloid progenitors through to neutrophil populations (band, segmented, mature) (Fig.1A-left). Ex vivo differentiated neutrophils retained transcriptional signatures reflective of effector functions (granule biogenesis, chemotactic activity, respiratory burst) and mapped to previously described human neutrophil populations in vivo. Ex vivo generated neutrophils showed expected functional properties of phagocytosis and neutrophil extracellular trap formation upon stimulation.
Exposure to IMiDs caused a maturation arrest with a 40-50% (p<0.001) decrease in abundance of differentiating metamyelocytes, and neutrophil populations. Crucially, this maturation arrest was due to an accumulation of transcriptionally distinct myeloid precursors that differentiated though an aberrant pathway (population 6, Fig 1A). Furthermore, immature erythroid clusters representing 10% of live cells accumulated specifically in IMiD-treated samples (p<0.001) in absence of erythropoiesis-stimulating cytokines.
Simultaneous profiling of gene expression and chromatin accessibility in individual cells confirmed the presence of distinct phenotypes for untreated and IMiD-treated myeloid cells (Fig.1A) enabling the characterisation of the genome landscape of myelopoiesis, which has previously proven challenging. As expected, we observed dysregulation of Ikaros targets including PU.1 upon IMiD treatment (Fig. 1B-frame). The ability to study distinct and aberrant cellular populations at transcriptional and genome regulation level in parallel allowed us to identify several novel regulators of normal and perturbed neutrophil development. For example, we observed perturbed activation patterns of the transcription factors (TFs) zinc finger protein (ZNF467) and interferon regulatory factor 5 (IRF5) and the expression of their downstream targets as illustrated in Fig.1B. ZNF467 orchestrates a transcriptional program supporting terminal neutrophil differentiation in untreated cells (Fig.1B-top right), whilst the regulatory repertoire of IRF5, a hallmark of neutrophil activation is confined primarily to treatment-specific immature populations (Fig.1B-bottom right).
We present a multiomic atlas of normal myelopoiesis which we believe will provide an important resource for the study or normal and perturbed neutrophil development. We use this platform to study IMiD-induced neutropenia and identify two aberrant cellular progenitor populations (aberrant myeloid precursors & erythroid progenitors) with distinct molecular properties as the likely cause of myelosuppression.
Disclosures
Simoglou Karali:BMS: Research Funding. Wen:AstraZeneca: Current Employment. Sousos:University of Oxford: Patents & Royalties: 2203947.3 ; AOP Orphan Limited: Other: Educational travel grant. Pierceall:BMS: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Thakurta:Antenegene, BMS: Consultancy, Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Honoraria, Research Funding. Gandhi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Hagner:BMS: Current Employment, Current equity holder in publicly-traded company. Mead:Relay Therapeutics: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; University of Oxford: Patents & Royalties: 2203947.3 ; Pfizer: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Alethiomics Ltd: Consultancy, Current equity holder in private company, Other: Cofounder & equity holder, Research Funding; Karyopharm: Consultancy, Speakers Bureau; Sierra Oncology: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; GSK: Consultancy, Speakers Bureau; Galecto: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Roche: Research Funding; Sensyn: Consultancy, Speakers Bureau; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Other: investigator for AbbVie sponsored trials, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal